Background and Significance:
Myelofibrosis (MF) is one of several myeloproliferative neoplasms (MPN) associated with somatic mutations in JAK2, CALR, or MPL. Thrombocytopenia is a common occurrence in patients with MF and associated with poor outcomes; the rate of moderate thrombocytopenia (platelet counts of <100 x 10 9 /L) in patients with JAK inhibitor (JAKi)-naïve MF is approximately 25%. While multiple JAKi therapies have been approved for MF, thrombocytopenia is an on-target side effect and suboptimal doses are often used in thrombocytopenic patients leading to suboptimal symptom and/or spleen response. Selinexor is an investigational oral XPO1 inhibitor that may inhibit MF-relevant pathways including STAT, extracellular signal-regulated kinase (ERK), and protein kinase B (AKT). Previously we reported that in a Phase 1 study of selinexor (using two different doses) and the JAKi ruxolitinib, the combination was generally tolerable and manageable in JAKi-naïve patients with MF (NCT04562389). In the cohort receiving 60 mg selinexor weekly in combination with ruxolitinib, 79% of the intent-to-treat (ITT) population achieved a ≥35% reduction in spleen volume from baseline (SVR35) at Week 24; SVR35 rates were consistent across subgroups, including gender and regardless of ruxolitinib starting dose (this includes patients treated with ruxolitinib 5mg BID, a dose associated with limited SVR and TSS responses). Robust symptom improvement was also observed with 58% of the ITT achieving a ≥50% reduction in total symptom score from baseline (TSS50) at Week 24. Additionally, a Phase 1 study of selinexor monotherapy in patients with MF refractory or intolerant to JAKi therapy (NCT03627403; ESSENTIAL) has reported a generally tolerable and manageable safety profile with improvements in SVR35. Single agent selinexor resulted in a robust rate of SVR35, achieved by 4 of 10 patients (40%) at ≥24 weeks; responses were durable with long term therapy beyond 2 years. Positive results of this monotherapy study provide rationale to further explore selinexor as a monotherapy in subpopulations with MF with high unmet need such as those with thrombocytopenia. XPORT-MF-044 will therefore evaluate the efficacy and safety of selinexor monotherapy (40 mg and 60 mg QW) in patients with JAKi-naïve MF and moderate thrombocytopenia in addition to assessing the performance of JAKi add-on treatments in those patients who achieve suboptimal response to selinexor monotherapy.
Study Design and Methods:
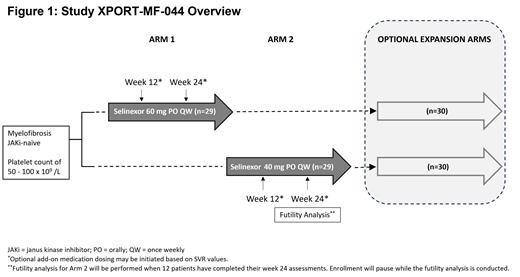
The XPORT-MF-044 trial is a two arm, sequential, multicenter, open-label, Phase 2 study with corresponding optional expansion to evaluate the efficacy of selinexor for up to 118 patients with JAKi-naïve MF and moderate thrombocytopenia ( Figure 1). Patients in Arm 1 will receive 60 mg of selinexor and those in Arm 2 will receive 40 mg of selinexor. Both arms will be able to enroll an additional 30 patients independently for the optional expansion arms after reaching 29 patients. Optional treatment of ruxolitinib or pacritinib, may be added for patients whose SVR is <10% at week 12 or <35% at week 24 when compared to baseline; and the choice of ruxolitinib or pacritinib will be considered based on platelet count. Select inclusion criteria are patients ≥18 years of age, measurable splenomegaly as demonstrated by spleen volume of ≥450 cm 3 by MRI or CT scan, dynamic international prognostic scoring system (DIPSS) of intermediate-1 with symptoms, intermediate-2, or high-risk, Eastern Cooperative Oncology Group (ECOG) performance status ≤2, platelet counts of 50 - 100 x 10 9 /L, and not eligible for stem cell transplantation. Major exclusion criteria are >10% blast in peripheral blood or bone marrow and previous treatment with JAKi for MF. The primary endpoint is SVR35 at week 24. Primary secondary endpoints are safety, TSS50 at week 24, anemia response at week 24, overall survival, and overall response rate. Efficacy and safety assessments will also be performed on patients who receive optional add-on treatment.
Disclosures
Gerds:AbbVie, Bristol Myers Squibb, Constellation Pharmaceuticals, GlaxoSmithKline, Kartos, Novartis, PharmaEssentia, Sierra Oncology: Consultancy; Accurate Pharmaceuticals, Constellation Pharmaceuticals, CTI BioPharma, Imago BioSciences, Incyte Corporation, Kratos Pharmaceuticals: Research Funding. Ritchie:Astellas Pharma, Jazz Pharmaceuticals, NS Pharma: Research Funding; Ariad: Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding; Novartis: Consultancy, Other: Travel Support, Research Funding, Speakers Bureau; Celgene: Other: Travel Support, Speakers Bureau; Celgene, Incyte Corporation, Novartis: Consultancy; Pfizer: Consultancy, Other: travel, Research Funding. Wang:Karyopharm: Current Employment. Kye:Karyopharm: Current Employment. Rampal:Karyopharm: Consultancy; Zentalis: Consultancy; Servier: Consultancy; Kartos: Consultancy; Morphosys/Constellation: Consultancy; Dainippon: Consultancy; Pharmaessentia: Consultancy; Constellation: Research Funding; CTI BioPharma Corp: Consultancy; Stemline: Research Funding; Zentalis: Research Funding; Incyte: Research Funding; Sumitomo: Consultancy; Galecto: Consultancy; Celgene-BMS: Consultancy; Ryvu: Research Funding; GSK-Sierra: Consultancy; Incyte: Consultancy.